Barium-coated areas of the digestive tract then appear on an X-ray as white allowing for greater visual detail than a traditional X-ray Figure 154. Learn vocabulary terms and more with flashcards games and other study tools.

Solved 3 Copper Ii Sulfate Sodium Phosphate Observations Chegg Com
Learn vocabulary terms and more with flashcards games and other study tools.

. The component ions in a salt compound can be either inorganic such as. In chemistry a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions which results in a compound with no net electric charge. A potassium phosphate b copperII sulfate c calcium chloride d titanium dioxide e ammonium nitrate f sodium bisulfate the common name for sodium hydrogen sulfate The following ionic compounds are found in common household products.
Ionic Equation Worksheet Write balanced molecular total ionic and net ionic equations for each of the following. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. In a total of 10000 lbs of sodium sulfate 3236 lbs are sodium 2257 lbs are sulfur and 4506 lbs are oxygen.
Aqueous potassium carbonate reacts with aqueous silver nitrate to precipitate silver carbonate. Start studying Module Two Chem 101 Problems. A CaH 2 PO 4 2 b FeSO 4 c CaCO 3 d MgO e NaNO 2 f KI.
Since the K sp of barium sulfate is 23 10 8 very little of it dissolves as it coats the lining of the patients intestinal tract. Uses of Ammonia In the production of urea and rayon and certain fertilisers such as ammonium nitrate urea diammonium phosphate ammonium sulfate and so on. In the preceding calculations the percent of each element in the substance was found.
Aqueous sodium hydroxide reacts with aqueous copperII sulfate to precipitate copperII hydroxide. In terms of a pound mass unit. The percent of groups of elements may also be calculated.
Sodium chloride is the salt. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. Name each of the compounds.
A common example is table salt with positively charged sodium ions and negatively charged chloride ions. Suppose you want percent sulfate SO 4 2 in sodium. In reaction with copper sulfate solution it gives a deep blue coloured complex compound tetraamminecopper rmII sulphate.
Start studying Chapter 6 Ionic and Molecular compounds.

Solved Copper Ii Sulfate Sodium Carbonate Observations Chegg Com

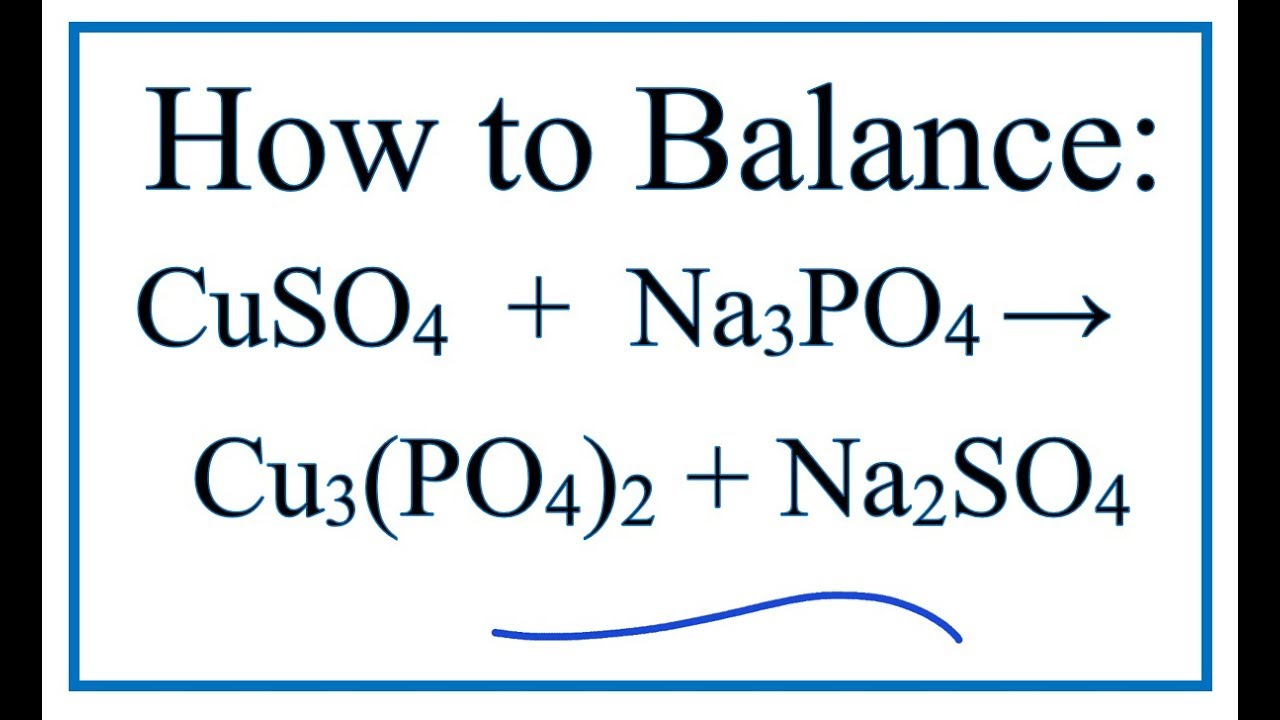
What Is The Molecular Ionic And Net Ionic Equations For Copper Ii Sulfate Sodium Phosphate Study Com
0 Comments